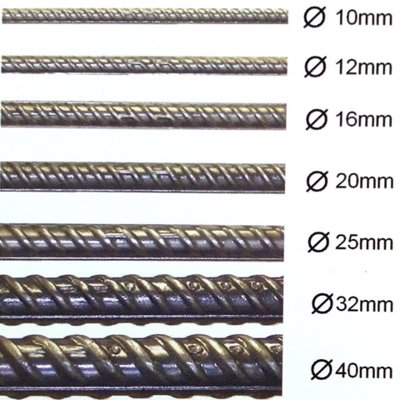
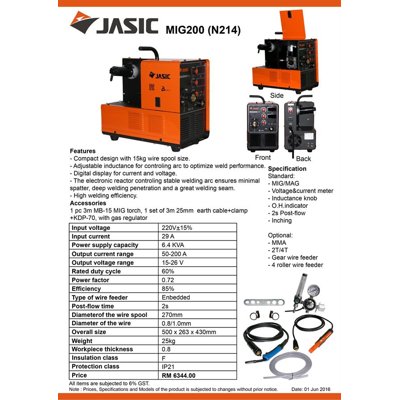
The New Way To Source For Construction, Interior And Home Materials
 Online Shopping
Online Shopping
 Request For Quotations
Request For Quotations
 Multi Channel Payment
Multi Channel Payment
Featured Suppliers
The Company has the goal to become a leader in furniture business, construction project, decoration products, and refurbishment of building with the provision of complete services as “South Island Furniture” to attain highest customer satisfaction. At present the Company has more than 60,000 items of products.
Find Out More
We also QC on the area of Finishing, Fixtures and Fittings.
Find Out More
Wide Range of Varieties
We have over 500 distinct designs and color variation make up our collection of more than 2000 products. Some of the design themes that are popular among our clients are Classic Elegance, Simply Natural, Modern Charm and many more.
Customizable
We can customize our products to suit your project requirements. Just tell us your preferred design and size, we will suggest the best that suit your room to improve the ambiance environment.
High Quality Materials
We use only materials of the highest quality manufactured in compliance with ISO 14001:2004 and ISO 9001:2008. Our products are fire retardant, resistance against light-fastness, washable and wear-resistant.
Find Out More
Find Out More
MolecQ distributes a diverse range of products such as decorative and commercial faucet, fittings, shower products, jacuzzi, sauna, basin, bathroom accessories and other sanitary ware products under the brand MODERN DEPOT, MODERN DEPOT SPAS and FILTON.
Find Out More
The key team consist of individuals with chemical engineering, accountant, and business administration background. Led by AFA and supported by LIN, CW have grow enormously within short time span with its brand being recognized by elite group within Malaysia, Brunei, and United Kingdom. Leading Islamic television station in Malaysia, TV Alhijrah have also engaged with CW for specially tailored business program while many big names in interior design, entertainment industry, mass media, business owner, and VVIP have started to engage with CW for its services and products.
Find Out More
Our brands of tools are well renowned for its quality and performance that allow end-users to complete jobs safely and reliably. All our products are covered with comprehensive warranty & dependable technical backup so as you can be at ease of mind with your purchases.
Find Out More
Visit Supplier's Page
Find Out More
Bringing these pieces of Art from all over the Orient.
Specialist in Persian, Oriental & Contemporary Hand Woven Carpets, Rugs & Kilims.
Also available wide selection of machine made carpets in popular designs.
Specialist in custom-made carpets for suites, hotels and residences, in-house designs available.
Find Out More
Garden Decorative Items
Solid Wood Decorative Items for Landscaping
Find Out More
because we have all the home improvement materials you need.
Purchasing a home is one of the largest investments that most people will make. This means that people want to ensure it is protected in every possible way. Any home will require an element of improvement at some point and this could include new kitchen cabinets, bathroom tiles or even paint. Many areas of the home also require updating to ensure safety and efficiency such as heating and cooling systems.Our varied list of products that we offer are aimed at assisting home improvements and renovations. This includes the Korean kitchen cabinet, bathroom accessories, kitchen appliances, shower screen, hardware, aluminum sliding door & window and tiles – all of which are designed to keep your home looking fresh and new.
Find Out More
Find Out More
You can trust tinted window films when it comes to residential glass cooling because they are built to keep a room cool without taking away a lot of sunlight. How? It prevents excessive amount of heat from getting into a particular space. If that is not enough, it also helps reduce the risk of dangerous UV rays from causing discolouration to your décor or paint and damaging your skin, amongst others.
Today, we present another type of tinted window film that has proven to do a better job than most window films in the market. Introducing Camo Window Films, a residential glass cooling component that not only caters to automobiles but also for home, office, or commercial spaces. It is solely distributed by Camo Marketing Sdn Bhd, a subsidiary company of Apple Auto Sdn Bhd (AASB).
Find Out More
In 1993, Versalink opened our first showroom in Sungai Buloh, Selangor. Our reputation soon grew by leaps and bounds even as our clientele pool increased. We have grown at such a rate that going global was made possible, we started our first export in 1993 too.
Today, Versalink is a premier Malaysian brand of high quality office furniture, It is also an esteemed trademark around the world. In the previous decade, we had exported about 80% of our quality office furniture to various countries all around the world.
Find Out More
Today we are proud to offer many furnitures that are of trendy designs to suit today’s fast changing requirements; allowing clients’ to express their individually through the different series – to reach for the stars as they say.
We also stock and sell furnitures that are classical in design for those who prefer a more conservative style.
All our furnitures are superbly crafted to be of superior quality and are long lasting. With a warehouse storage capacity of well over 70,000 square feet, stocks are easily available, and we are able to serve our clients better.
Visit Supplier's Page
Find Out More
Find Out More
"what is useful can be beautiful, what is beautiful can be affordable"
Find Out More
With more greens around us improves the air quality and create the mood and energy of human beings. Last but not least, we do play an important role in preserving the “Save the environment” in the world that we live in!
Find Out More
Find Out More
Our range of products from budget to high-end are of the best quality at wholesale and outlet prices.
We carry wide selections of modern, retro pendant light, Ceiling lamps, chandeliers, downlight, floor lamps, led light, light bulb, outdoor lamps, pendant lamps, table lamps, wall lamps.
Find Out More
Door World can provide a surveying service by carrying out an onsite consultation process which is carefully designed to establish customers’ expectation, discuss ideas and provide the best value solution at competitive prices that would provide ultimate customers’ satisfaction and peace of mind.
Find Out More
Find Out More
In 2010, Best & Chunlin was proud to announce the collaborations with distinguished furniture brands from Europe, like Artisan, Prostoria, KOO and Gamamobel.
In 2014, The Tekni International had been established under Best & Chunlin Company. The new Brand is created to cater to the different market need and to allow customers a wider variety of furniture models to choose from. The founder of Best & Chunlin, Mr. Chun Lin Yu strongly believes that “ the perfect products under the diffusions range worldwide can bring to customers and their families the perfect and exclusive living space that everyone strives to have. This is hard to be done by any single furniture brand”. This belief is from his passion and experiences that had spanned for more than 45 years in furniture design and sales.
Tekni has stores in Taiwan, Shanghai, Wuxi and Malaysia. In the near future Tekni aims to launch more stores. Tekni only selects and stocks the carefully crafted modern furniture that display not only beautiful designs but also classic and luxurious style.
Find Out More
The company is dedicated to source premium kitchen & sanitary ware for fellow Malaysians, which includes a joint effort of offering world class brands. These reputable brands are Blanco, Roca, Abagno, Grohe, Johnson Suisse and SSWW. We are determined to keep our brand portfolio wide and equipped with the latest and most innovative products in the industry.
Find Out More
The company has expanded the export market to various regions, from South East Asia, Middle East to internationally.
Driven by our passion, we are dedicated to enhance the office environment into extra flexible and structured as well as creating and improving workplace solutions.
In promising with the values that grow at the heart of our business philosophy, Classic Chair System Sdn Bhd’s objectives are continually strive and innovate in manufacturing and exporting our unique world-class office furniture.
Find Out More
Find Out More
Brand Mall by BMO
Building & Construction
Fitting & Furnitures
Latest Products
Articles
Brands available in BMO